TOPIC 12: Drug Allergy (FEATURED READING) – Drug Allergy Practice Parameter Updates to Incorporate Into Your Clinical Practice
OFFICIAL ABP TOPIC:
Drug Allergy (FEATURED READING) – Drug Allergy Practice Parameter Updates to Incorporate Into Your Clinical Practice
BACKGROUND
Drug allergy affects up to 10% of the population and can lead to significant morbidity and mortality. This review summarizes key changes in the 2022 drug allergy practice parameter update to help clinicians more accurately diagnose and manage drug allergies.
ANTIBIOTIC HYPERSENSITIVITY
PENICILLIN ALLERGY
- Over 95% of patients with reported penicillin allergy are not truly allergic.
- The penicillin allergy label is associated with negative consequences such as increased costs, antibiotic-associated infections, and antibiotic resistance.
- Proactive delabeling of penicillin allergy should be done, when possible, especially in elective settings.
- Direct amoxicillin challenge is preferred for children with history of benign cutaneous reactions (e.g., morbilliform drug eruption, urticaria) without systemic symptoms.
- Testing is not needed to delabel a child with history of a reaction inconsistent with penicillin allergy (e.g., headache), but an amoxicillin challenge may be offered to families nervous about removing the penicillin allergy label.
- Penicillin skin testing is recommended for patients with a history of anaphylaxis or recent penicillin reaction that is likely IgE-mediated.
- Positive skin test: Avoid penicillin.
- Negative skin test: Confirm negative test with amoxicillin challenge.
- Do not use multiple-day amoxicillin challenges; a 1- or 2-step challenge is sufficient.
CEPHALOSPORIN ALLERGY
- Cephalosporin allergy affects 0.5–2% of patients.
- Allergic cross-reactivity is highest with cephalosporins sharing identical R1-group side chains.
FIGURE 1
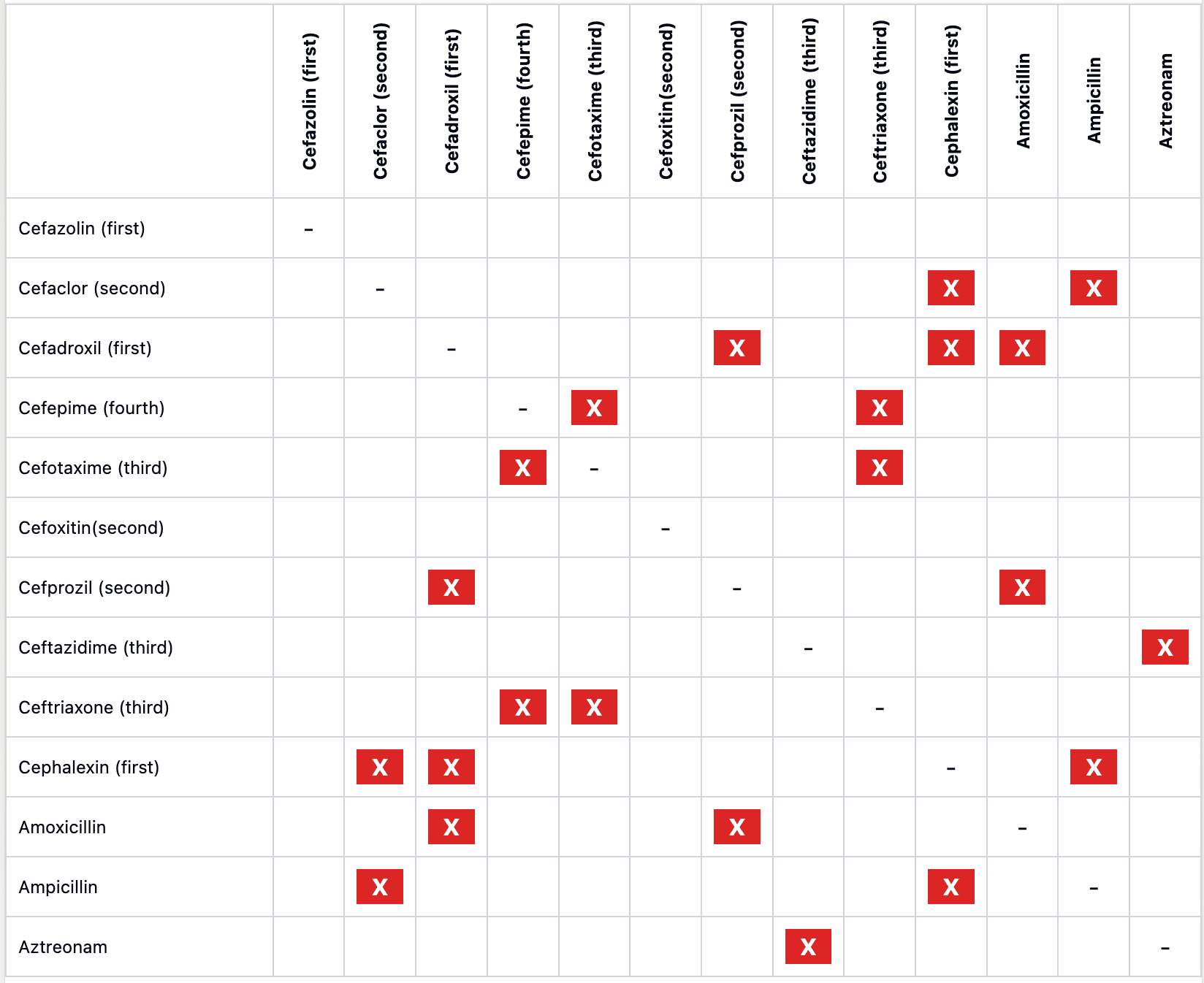
- To evaluate a potential cephalosporin allergyin a child anticipated to need treatment with cephalosporins:
- If history of nonanaphylactic reaction: Administer cephalosporin with dissimilar side chain.
- If history of anaphylactic reaction: Skin test with dissimilar parenteral cephalosporin before administration.
- Positive skin test: Avoid antibiotic.
- Negative skin test: Confirm negative testing with oral cephalosporin challenge.
- Nonirritating concentrations are available for several cephalosporins (see Table 2).
- If negative, proceed to drug challenge; if positive, use alternative or desensitize.
BETA-LACTAM CROSS-REACTIVITY ALGORITHM
Beta-lactam cross-reactivity is rare; only 2% of patients with penicillin allergy cross-react to cephalosporins. Figure 3 in the featured reading includes a flow diagram summarizing:
- Cephalosporin administration to penicillin-allergic patients:
- Anaphylactic penicillin allergy: Can administer structurally dissimilar cephalosporin without testing (cefazolin has a unique side chain).
- Nonanaphylactic penicillin allergy: Can administer any cephalosporin without testing.
- History of penicillin-induced anaphylaxis and need for structurally similar cephalosporin:Perform penicillin skin testing.
- Skin test negative: Can administer cephalosporin normally.
- Skin test positive: Administer cephalosporin via 2-step graded challenge or induction of tolerance.
- Penicillin administration to cephalosporin-allergic patients:
- Nonanaphylactic cephalosporin allergy: Can administer penicillin without testing.
- Anaphylactic cephalosporin allergy: Perform penicillin skin testing. If negative, do a penicillin challenge.
- For patients with any penicillin or cephalosporin allergy:
- Can receive carbapenems without testing (extremely low rate of cross-reactivity).
- Can receive aztreonam without testing, unless history of ceftazidime allergy (same R1 side chain).
- Avoid ceftazidime in aztreonam-allergic patients.
SULFONAMIDE ANTIBIOTICS
If there is a need to remove the sulfonamide allergy label:
- For benign cutaneous reactions (urticaria, morbilliform drug eruption) >5 years ago, perform 1-step trimethoprim-sulfamethoxazole challenge to remove the allergy label.
- Avoid challenging/desensitizing patients with a history of severe cutaneous adverse reactions.
FLUOROQUINOLONES AND MACROLIDES
- For nonanaphylactic reactions >5 years ago, consider a 1- or 2-step challenge with the drug.
- For more recent or severe reactions, consider tolerance induction or a 2-step challenge with a different fluoroquinolone.
NSAID HYPERSENSITIVITY
TABLE 1: COX-1 AND COX-2 INHIBITING MEDICATIONS
|
Drug Category |
Drugs |
|
Highly selective COX-1 inhibitors |
Acetylsalicylic acid, diclofenac, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefenamic acid, naproxen, oxaprozin |
|
Weakly selective COX-1 inhibitors |
Acetaminophen, choline magnesium trisalicylate, diflunisal, salsalate |
|
Preferentially selective COX-2 inhibitors |
Meloxicam, nabumetone |
|
Highly selective COX-2 inhibitors |
Celecoxib |
- A selective COX-2 inhibitor (celecoxib) can be used as an analgesic in patients with COX-1 hypersensitivity.
- Most children with NSAID hypersensitivity will tolerate NSAIDs when challenged.
- For patients with a history of NSAID-induced urticaria/angioedema, a 2-step aspirin challenge can identify if COX-1 cross-reactive.
- For benign delayed hypersensitivity reactions (e.g., fixed drug eruption), consider patch testing at the site of past reaction or challenge with a dissimilar NSAID.
- For severe reactions, avoid NSAIDs as testing is not reliable.
TESTING FOR DELAYED HYPERSENSITIVITY REACTIONS
Delayed hypersensitivity reactions occur at least 6 hours after a dose, and usually 1-2 weeks after starting a medication.
- May consider delayed intradermal testing (IDT) or patch testing (PT)for:
- Morbilliform drug eruption (6 weeks to 6 months after reaction).
- Drug reaction with eosinophilia and systemic symptoms (6 months after reaction and at least 4 weeks off steroids).
- Acute generalized exanthematous pustulosis.
- Testing helps determine drug causality and cross-reactivity.
- Use the highest nonirritating drug concentration.
- Initial PT readings at 48 hours, repeat at 96 hours and 7 days.
- IDT readings at 24-48 hours.
- For milder exanthems or low-risk distant reactions, consider:
- Treating through initial rash.
- Graded drug reintroduction after discontinuation.
- 1- or 2-step drug challenge.
- Avoid drug challenges for severe reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis, or drug-induced organ failure.
REACTIONS TO CHEMOTHERAPEUTICS AND BIOLOGICS
- For immediate hypersensitivity reaction to chemotherapeutic:
- Stratify risk by initial reaction severity and skin testing (if available) to guide management.
- Consider desensitization if the drug is the preferred therapy.
- For nonimmediate or inconsistent reaction to chemotherapeutic:
- Readminister with slower infusion, dose escalation, or premedications.
- For biologics(e.g., monoclonal antibodies):
- Skin testing is limited due to high cost and uncertain predictive value.
- For nonimmediate or inconsistent reactions:
- Readminister with slower infusion, dose escalation, or premedications.
- For immediate hypersensitivity reaction/anaphylaxis:
- Consider desensitization if the drug is the preferred therapy.
